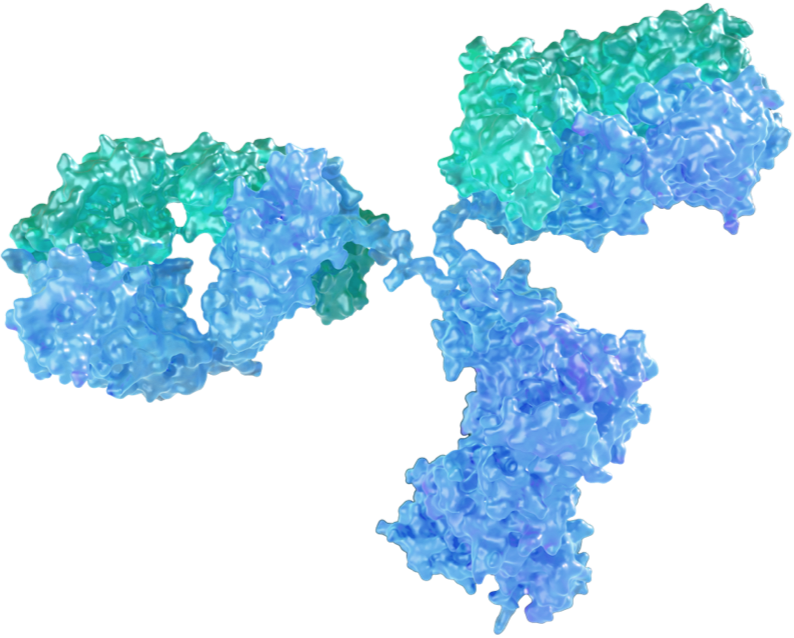
MAU868 is the first neutralizing antibody targeting the BK Virus (BKV). BKV is a polyoma virus that can be reactivated when a patient is immunocompromised, such as in kidney transplant and hematopoietic stem cell transplant (HSCT) recipients.
Vera holds an exclusive worldwide license for the development and commercialization of MAU868 in all indications from Pfizer.



Recently Completed Ph2 study to evaluate efficacy and safety of MAU868 in the treatment of BK viremia in kidney transplant recipients