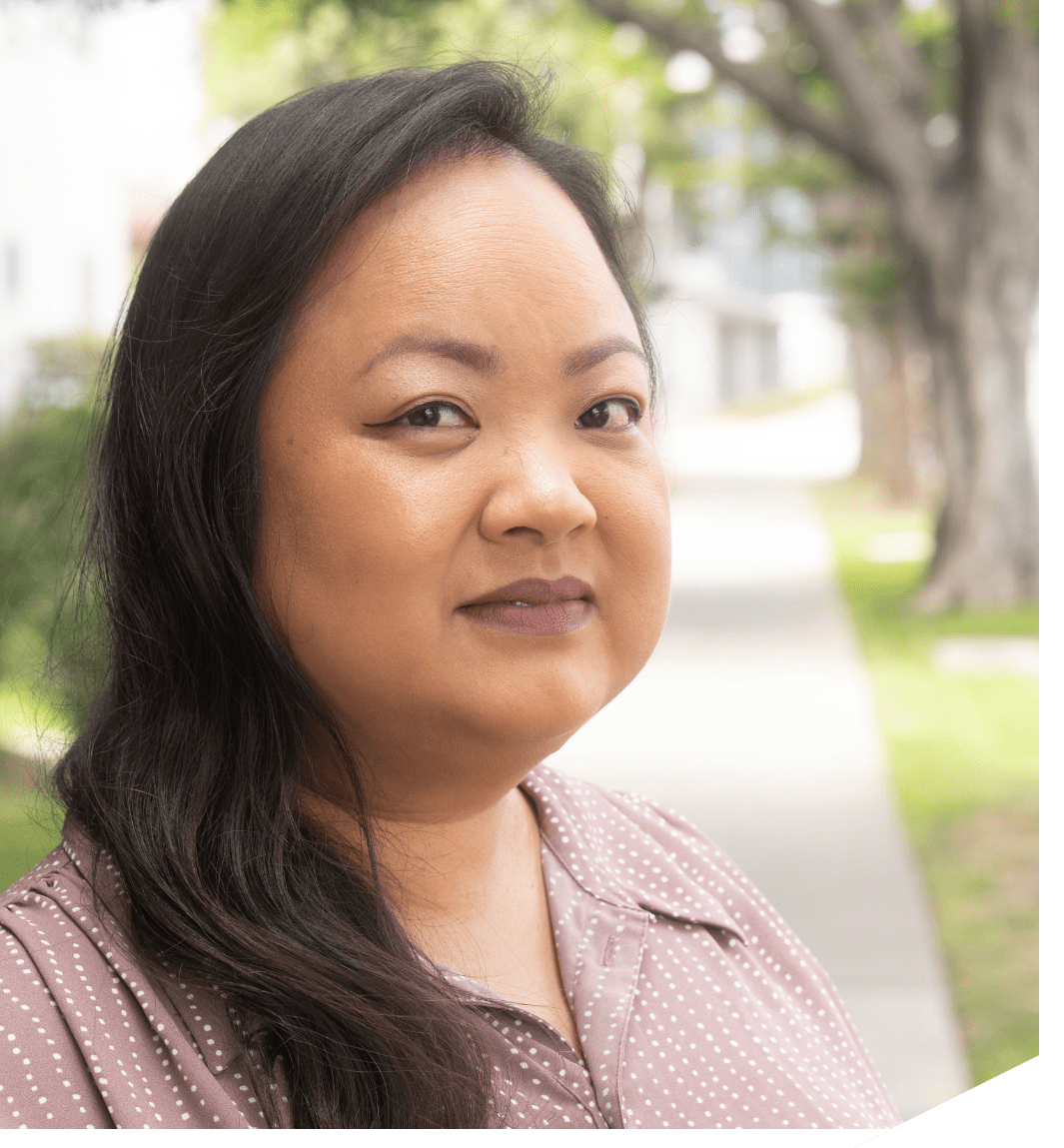
IgA Nephropathy has reframed my life—entirely.
At 22 years old, while going to college and working full-time, I noticed black spots in my vision. When I went to the doctor he told me it was likely just stress – but he reluctantly agreed to run some tests. The next morning, he called and told me to go to the emergency room immediately.“